Direct naar Forum

|
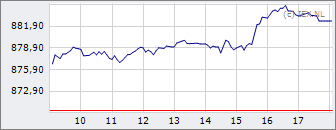
AEX
870,27
-3,75
-0,43%
18:05
|
|
|
|

|
Germany40^ |
17.943,40
|
-0,80%
|
|

|
BEL 20 |
3.857,94
|
-0,67%
|
|

|
Europe50^ |
4.957,00
|
-0,66%
|
|

|
US30^ |
38.124,13
|
-0,60%
|
|

|
Nasd100^ |
17.464,90
|
-0,34%
|
|

|
US500^ |
5.054,93
|
-0,34%
|
|

|
Japan225^ |
37.725,14
|
-0,72%
|
|

|
Gold spot |
2.335,68
|
+0,84%
|
|

|
EUR/USD |
1,0734
|
+0,34%
|
|

|
WTI |
83,77
|
+1,05%
|
|
#/^ Index indications calculated real time, zie
disclaimer